India’s comprehensive ban on electronic nicotine delivery systems (ENDS), including e-cigarettes and vaping devices, stems from decisive public health priorities and regulatory concerns.
Core Rationale for the Ban
- Youth Epidemic Prevention: The Indian government identified aggressive marketing and flavored products targeting youth as a major threat, fearing a new generation could become addicted to nicotine and potentially transition to combustible tobacco.
- Lack of Regulatory Framework & Safety Evidence: Unlike medicines or regulated tobacco products, there was no established domestic regulatory mechanism for ENDS. Health authorities cited insufficient long-term evidence on their safety and efficacy for cessation, particularly concerning lung and cardiovascular impacts. Concerns included poor manufacturing standards and unverified nicotine levels.
- Gateway to Tobacco Use: Authorities viewed vaping as a potential gateway, particularly for non-smoking youth, towards using traditional combustible tobacco products, undermining decades of tobacco control efforts. Dual use (vaping + smoking) was also a significant concern.
- Preserving Existing Tobacco Control Gains: Banning ENDS aligned with India’s strong stance on reducing tobacco consumption under the Cigarettes and Other Tobacco Products Act (COTPA) and WHO FCTC obligations. Introducing a new nicotine product was seen as counterproductive.
- Public Health Emergency Mindset: Former Health Minister Dr. Harsh Vardhan framed it as a “decisive step to protect health,” reflecting a precautionary approach prioritizing immediate harm prevention over potential (and contested) relative harm reduction arguments.
Official Stance and Enforcement
The Prohibition of Electronic Cigarettes Act (2019) criminalizes the production, manufacture, import, export, transport, sale (including online), distribution, advertising, and storage of ENDS. Penalties include imprisonment (up to 3 years) and fines.
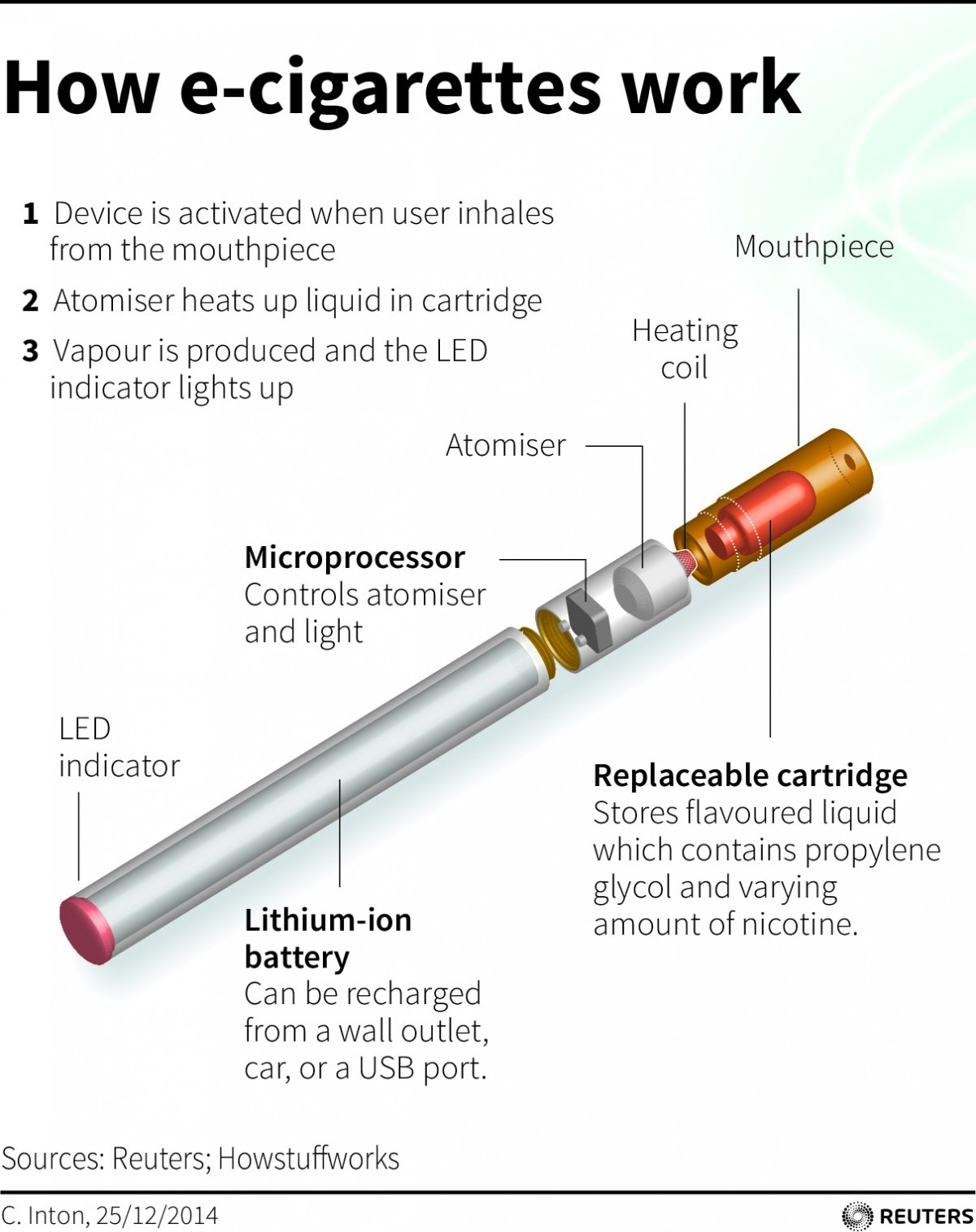
Health Minister’s Warning: “These novel products come with attractive appearances and multiple flavors and their use among youth, children and pregnant women is equally alarming.”
Underlying Context
- Massive Tobacco Burden: India faces a colossal public health crisis with extremely high rates of tobacco use (smoking and smokeless), leading to over 1.35 million tobacco-related deaths annually.
- Vape Industry’s Unregulated Entry: ENDS entered the market without prior regulatory approval or safety testing, creating an immediate perceived risk.
- WHO Guidance: India’s decision echoes WHO recommendations urging member states to regulate or ban ENDS to prevent youth uptake and non-smoker initiation, especially where comprehensive bans on tobacco advertising exist.
The ban represents a controversial public health choice favoring stringent control and youth protection over harm reduction arguments, citing the absence of definitive safety proof and vulnerability of India’s massive youth population.










