The regulatory landscape for electronic nicotine delivery systems (ENDS) underwent profound changes between the 2014 framework and the subsequent rules implemented primarily after 2016.
2014 Regulatory Landscape (Pre-Deeming)
Prior to 2016, regulation was fragmented and limited:
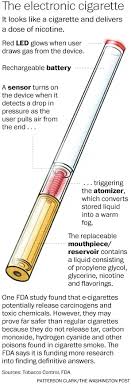
- No FDA Authority: ENDS were not classified as tobacco products under the Tobacco Control Act.
- Minimal Federal Oversight: Only products marketed for therapeutic purposes fell under FDA jurisdiction as drugs/devices.
- State/Local Focus: Regulation occurred primarily at state and local levels, focusing on youth access restrictions and indoor use bans.
- No Standardization: No federal requirements existed for manufacturing standards, ingredient disclosure, or health claims verification.
Transformative Shifts: The New Regulatory Era (Post-Deeming Rule & Beyond)
The FDA’s 2016 “Deeming Rule” fundamentally altered ENDS oversight, with critical updates continuing through subsequent years:
- Product Classification (Deeming Rule): ENDS are regulated as tobacco products under the Family Smoking Prevention and Tobacco Control Act.
- Premarket Authorization (PMTA): Mandatory pathway requiring manufacturers to demonstrate public health benefit before marketing. Existing products required retrospective applications. This is the most significant regulatory hurdle.
- Ingredients & Health Claims:
- Mandatory listing of all ingredients.
- Prohibition on modified risk claims (e.g., “safer than cigarettes”) without explicit FDA authorization.
- Required reporting of harmful/potentially harmful constituents (HPHCs).
- Age Verification & Sales Restrictions:
- Federal minimum purchase age raised to 21 nationwide (Tobacco 21 law, 2019).
- Enhanced age verification requirements for both online and retail sales.
- Restrictions on vending machine sales and self-service displays.
- Flavors:
- Enforcement prioritized against unauthorized flavored cartridge-based ENDS (excluding tobacco/menthol) appealing to youth.
- Marketing Authorization Required: Companies must gain PMTA authorization for any flavored product to remain on market.
- Manufacturing Standards & Compliance: Subject to FDA inspection and manufacturing practice regulations.
Key Changes Summarized
The evolution moved from limited oversight to a comprehensive framework defined by:
- FDA Jurisdiction: From virtually none to primary federal authority.
- Market Entry: From largely unregulated to requiring premarket authorization proving public health benefit.
- Transparency: Mandatory ingredient and HPHC reporting, replacing lack of disclosure.
- Youth Focus: Significantly stricter age verification, sales restrictions (T21), and targeted enforcement against youth-appealing flavors.
- Substantive Requirements: Introduction of manufacturing standards and marketing restrictions absent in 2014.