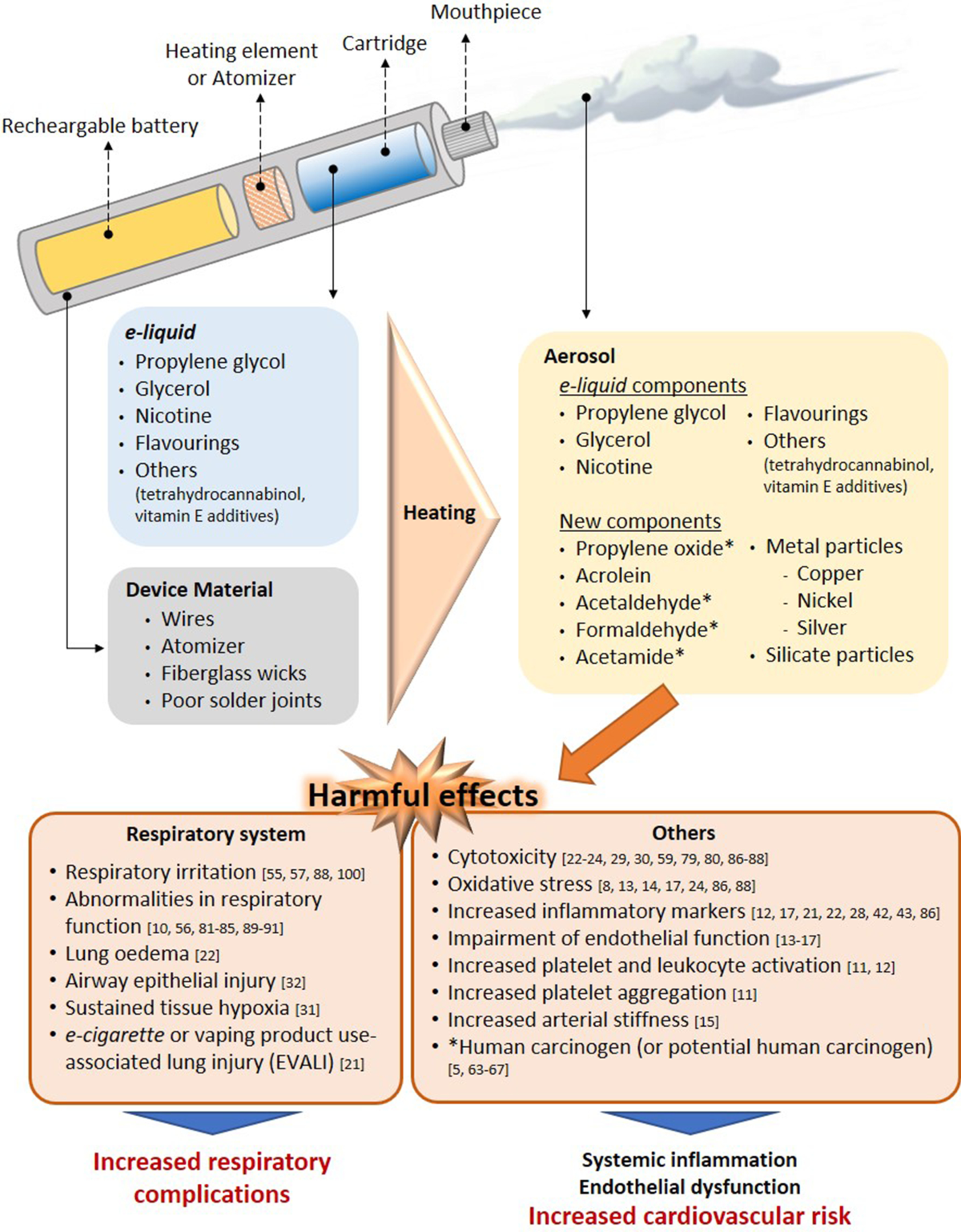
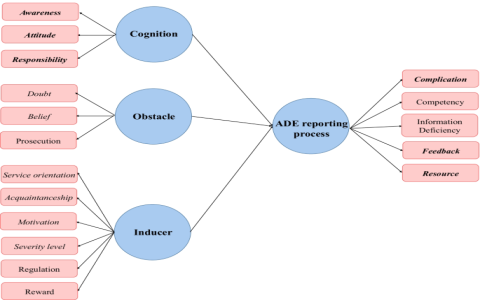
Effectively managing adverse events for e-cigarettes, such as respiratory issues or device malfunctions, is essential for minimizing risks and enhancing consumer safety. A streamlined reporting process ensures timely interventions and regulatory compliance.
Simple Steps for Effective Reporting
- Step 1: Recognize and Document – Immediately identify any adverse event, including symptoms, device details, and user information. Record specifics like date, time, location, and severity in a structured log.
- Step 2: Compile Comprehensive Data – Gather all relevant evidence, such as product lot numbers, usage patterns, and affected individuals’ demographics. Ensure anonymity where required to protect privacy.
- Step 3: Report to Authorities – Submit the documented event to designated regulatory bodies, such as national health agencies or e-cigarette manufacturers, using standardized reporting forms for accuracy.
- Step 4: Implement Follow-Up Actions – Track the incident resolution, communicate updates to stakeholders, and analyze trends to prevent recurrence through improved quality controls or user guidance.
Adhering to these steps fosters proactive risk management and supports continuous safety enhancements in the e-cigarette industry.