The U.S. Food and Drug Administration (FDA) does not grant general market “approval” for electronic cigarettes (e-cigarettes) as a category.
Regulatory Status Through the PMTA Process
E-cigarettes fall under the FDA’s tobacco product regulations. Manufacturers must submit a Premarket Tobacco Product Application (PMTA) to legally market a new tobacco product, including e-cigarettes, in the U.S. The FDA evaluates these applications based on public health standards.
Current Authorization Status
- No General Approval: The FDA has not approved any e-cigarette as a “modified risk tobacco product” or a smoking cessation device.
- Limited Marketing Orders: The FDA has granted Marketing Granted Orders (MGOs) for specific e-cigarette products. This means those particular products can be legally marketed in the US based on their submitted PMTA evidence.
- Denials: The FDA has issued Marketing Denial Orders (MDOs) for millions of products whose applications did not demonstrate sufficient public health benefit or raised youth access/use concerns, effectively banning those products.
Authorized Products
As of now, the FDA has issued MGOs only for a limited number of specific e-cigarette devices and e-liquid flavors, primarily:
- RJ Reynolds Vapor Company: Vuse Solo, Vuse Ciro, Vuse Vibe devices and related tobacco-flavored pods/cartridges.
- NJOY: NJOY Daily disposable e-cigarettes (tobacco flavor only), NJOY ACE device and related tobacco-flavored pods.
Important: MGOs are specific to exact products. Flavored products (especially menthol, fruit, candy) largely lack authorization. Numerous applications, including for major brands and many flavored products, remain under review.
Ongoing Enforcement and Risks
- Illegal Products: Many e-cigarettes sold in the US, particularly flavored disposable devices from various manufacturers, lack FDA marketing authorization and are illegally on the market.
- FDA Enforcement: The FDA actively issues warning letters, civil money penalty complaints, and injunctions against manufacturers and retailers selling unauthorized products, prioritizing enforcement against flavored cartridge-based e-cigs, flavored disposable products appealing to youth, and companies that didn’t submit timely PMTAs.
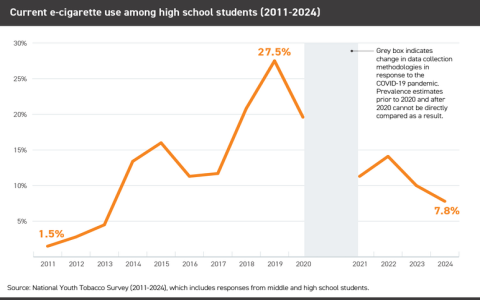
- Youth Usage: Preventing youth access, initiation, and use remains a primary FDA concern driving enforcement priorities.
Consumer Considerations
- FDA Authorization ≠ Safety: An MGO means the FDA determined the product is “appropriate for the protection of public health” relative to smokers switching completely, not that the product is safe. All tobacco products carry risks.
- Check for Authorization: The FDA publishes lists of products with MGOs and MDOs.
- Unauthorized Products Pose Risks: Unauthorized products have not undergone FDA review. Risks include potentially harmful ingredients and inconsistent nicotine delivery.