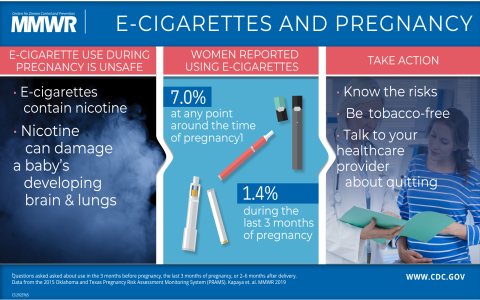
The FDA regulates electronic nicotine delivery systems (ENDS), including e-cigarettes, under its authority for tobacco products. However, it’s crucial to understand the specifics of FDA authorization versus full approval.
Regulatory Framework
The FDA does not “approve” tobacco products in the same way it approves drugs or medical devices. Instead, it authorizes them for sale in the US market through pathways like Premarket Tobacco Product Applications (PMTAs). Authorization signifies the product is “appropriate for the protection of public health” relative to existing tobacco products.
Current Authorization Status
- Limited Authorizations: The FDA has granted marketing granted orders (MGOs) authorizing specific e-cigarette products and devices. This number remains extremely small relative to applications submitted.
- Pre-Market Review Mandate: Legally, no new tobacco product (including e-cigarettes introduced after February 15, 2007) can be marketed without FDA authorization. Many products currently on the market lack authorization and are illegal.
- Focus on Youth Prevention: Authorizations heavily weigh the product’s impact on youth initiation and use. The FDA denies applications if marketing fails to demonstrate sufficient protection for youth.
Key Distinction: Authorization vs. Therapeutic Approval
No e-cigarette is FDA-approved as a smoking cessation aid. The FDA authorizes specific e-cigarettes as tobacco products, not as therapeutic devices or medicines. Smoking cessation claims require separate drug approval pathways, which no e-cigarette has achieved.
In summary, a very limited number of specific e-cigarette products have received FDA authorization to be legally marketed as tobacco products in the US. However, the vast majority on the market remain unauthorized, and none are FDA-approved for cessation.