India’s 2019 legislation imposed a comprehensive ban on the production, manufacture, import, export, transport, sale, distribution, storage, and advertisement of all Electronic Nicotine Delivery Systems (ENDS) and Heat-Not-Burn products. The primary justifications cited were:
- Protecting Youth: Addressing the rising use among adolescents and young adults.
- Public Health Precaution: Citing inconclusive evidence on long-term health effects and harm reduction relative to combustible tobacco.
- Gateway Concerns: Fears that e-cigarettes could lead to traditional tobacco use.
- WHO Guidance: Aligning with Framework Convention on Tobacco Control (FCTC) recommendations.
Global Regulatory Landscapes
Countries exhibit diverse approaches to e-cigarette regulation, ranging from complete bans to open access with varying levels of control:
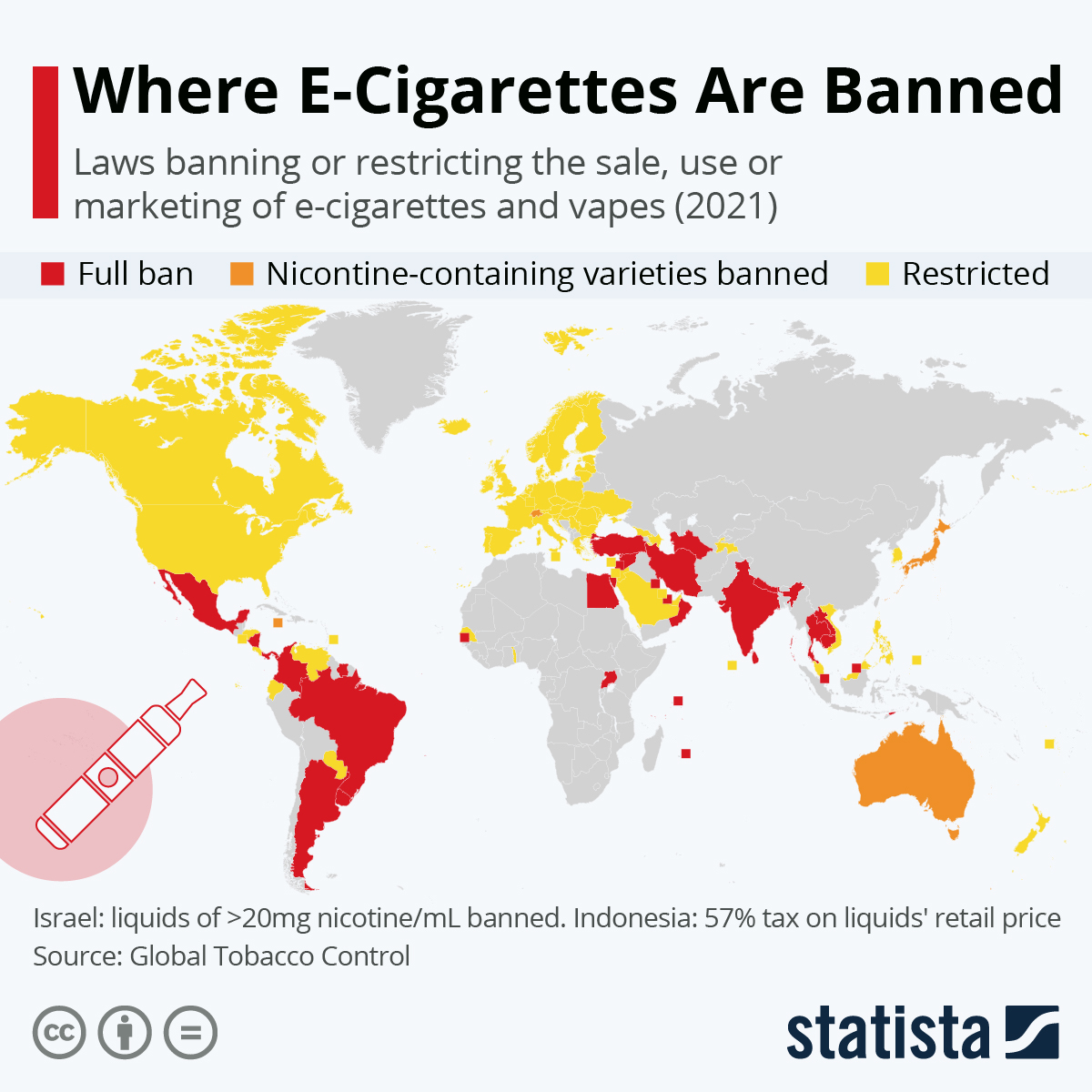
- Strict Prohibition (Similar to India):
- Brazil: ANVISA maintains a ban on the sale, import, and advertising of nicotine-containing e-cigarettes.
- Thailand: Possession, use, and sale are illegal, with strict penalties enforced for violations.
- Singapore: A long-standing ban under the Tobacco Act, covering sales, importation, and distribution.
- Uruguay & Argentina: Implemented national bans on sales.
- Regulated Legal Markets:
- United Kingdom: Employs a public health-oriented approach, regulating e-cigarettes as consumer products (MHRA). Promotes vaping as a significantly less harmful alternative for adult smokers seeking to quit, while restricting sales to minors, limiting nicotine concentration (20mg/ml max), and controlling advertising/packaging.
- European Union: The Tobacco Products Directive (TPD) sets harmonized rules across member states (excluding bans), including product safety standards, nicotine limits (20mg/ml), packaging/warning requirements, and marketing restrictions. Specific regulations (like flavor bans) vary by country (e.g., Netherlands banning non-tobacco flavors).
- United States: The FDA regulates e-cigarettes as tobacco products under the Tobacco Control Act. Manufacturers must obtain Pre-Market Tobacco Application (PMTA) authorization for new products. Sales to minors are illegal. Flavor restrictions are complex: a federal ban exists on most cartridge/pod-based flavors (except tobacco and menthol), while disposable flavors remain widely available. Significant enforcement challenges persist.
- Canada: Legal sale regulated under the Tobacco and Vaping Products Act (TVPA), including restrictions on nicotine concentration, flavors (provincial variations exist), packaging, and youth access.
- New Zealand: Previously adopting a harm reduction stance accessible by adults, recent policy shifts focus on reducing youth access (e.g., banning most disposable vapes and non-pharmacy sales).
Key Comparison Points
- Rationale: Banning countries (India, Singapore, Thailand, Brazil) prioritize the precautionary principle and youth protection. Regulating countries (UK, EU, Canada) often employ a relative harm reduction approach for adult smokers while mitigating risks.
- Scope: India’s ban is exceptionally broad, covering possession for use. Other bans typically target commercial activities (sales, import).
- Enforcement: Effectiveness varies significantly across jurisdictions with bans, with illicit markets posing challenges (India, Thailand). Regulated markets focus on compliance with product standards and access restrictions.
- Harm Reduction Recognition: Countries like the UK explicitly recognize the potential of regulated e-cigarettes as less harmful alternatives for adult smokers within comprehensive tobacco control strategies.










