Whether vaping devices are classified as tobacco products depends primarily on their nicotine source and regulatory definitions.
Key Regulatory Definitions
- U.S. FDA classification: E-cigarettes containing nicotine derived from tobacco are regulated as tobacco products under the Family Smoking Prevention and Tobacco Control Act.
- Nicotine-free vapes: Products without nicotine or tobacco derivatives generally fall outside tobacco regulations but may be subject to consumer safety laws.
Critical Composition Facts
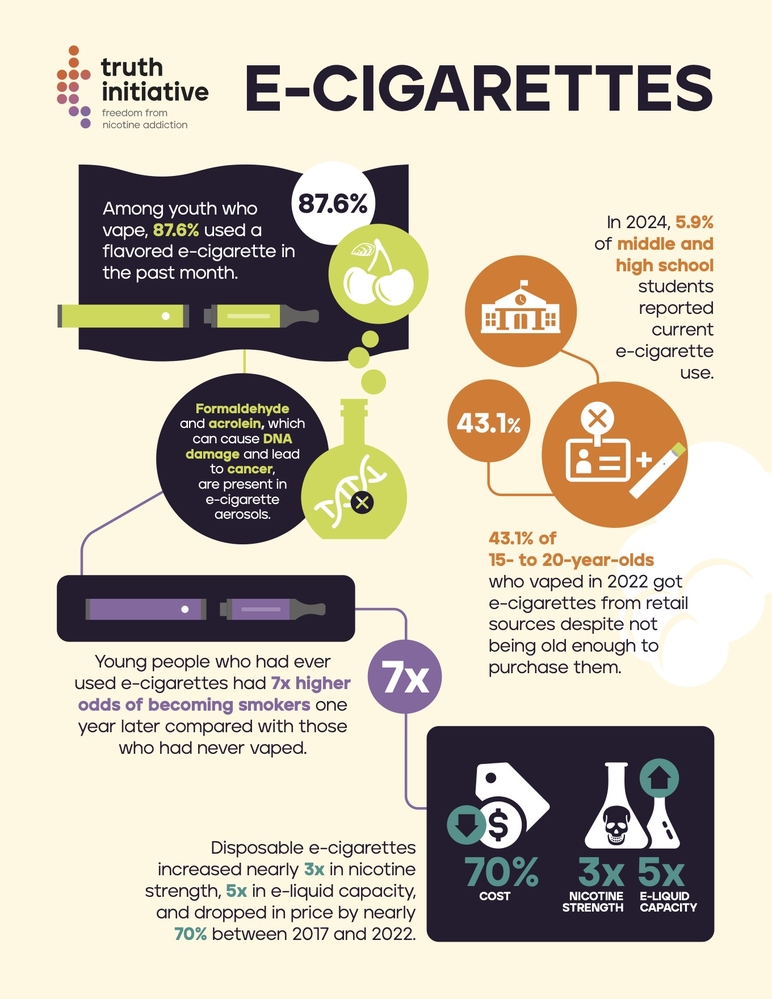
- Most vape liquids contain nicotine extracted from tobacco leaves, making them tobacco-derived products regardless of final form.
- Non-tobacco nicotine (NTN) synthesized in labs creates regulatory gray areas, though the FDA now asserts authority over synthetic nicotine products.
Legal and Health Implications
- Restrictions: Tobacco designation subjects vaping to age verification, marketing limits, and pre-market authorization requirements.
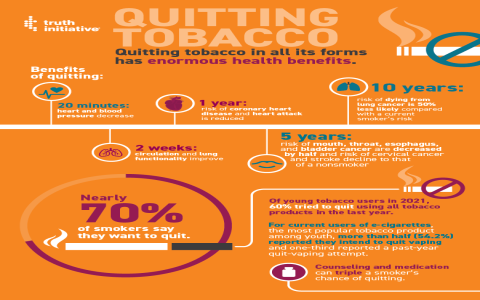
- Public health impact: While distinct from combustible cigarettes, vaping’s tobacco classification acknowledges nicotine addiction risks and youth prevention concerns.
International Variations
- EU: Regulates e-cigarettes under Tobacco Products Directive (TPD) regardless of nicotine content.
- Australia: Requires medical prescriptions for nicotine-containing vapes, treating them as therapeutic goods.
This classification affects product accessibility, taxation, and public health policies worldwide.